Case Study

Iddo Weiner
|
Dec 3, 2025
Overview
Modern antibody programs don’t just compete on biology: they compete on intellectual property strategy. Two IP-driven goals show up repeatedly in pharma and biotech: (1) patent estate expansion and (2) design-around / freedom-to-operate (FTO) discovery.
Patent estate expansion (what we refer to as “IP expansion”) is pursued by companies that already hold a lead antibody patent and want to broaden and strengthen their claims with additional, patentable sequence variants that preserve performance while widening coverage against future competitors.
Design-around / FTO discovery (what we call “IP escape”) is pursued by teams entering a space with existing patents, aiming to identify antibodies that are sufficiently different from their patented origin and can achieve similar therapeutic intent while supporting a clear freedom-to-operate path.
ConvergeAB™ addresses both needs through the same core capability: generative antibody design that explores highly diverged, non-intuitive sequence space while maintaining strong binding and developability profiles. Whether the goal is to expand a portfolio around an existing asset or to discover alternative molecules that enable competitive participation in a disease area, ConvergeAB™ provides a systematic, scalable way to generate novel, development-ready candidates aligned with the realities of today’s patent landscape.
Redefining the Boundaries of Antibody Design
In wake of both the recent epidemics and advancements in AI: speed and scalability became essential. Traditional antibody optimization methods, while precise, are resource-intensive and limited in scope. ConvergeAB™ introduces a new paradigm: using AI to navigate sequence space at scale, identifying variants that balance affinity, manufacturability, and developability simultaneously.
“We didn’t just optimize an existing sequence - we broadened the intellectual and biological landscape for therapeutic design, enabling a diverse set of new inventions with superior traits.”
—
Iddo Weiner, Chief Scientific Officer & Co-Founder at Converge Bio
The Experiment: Expanding IP Through Generative Design
Methodology
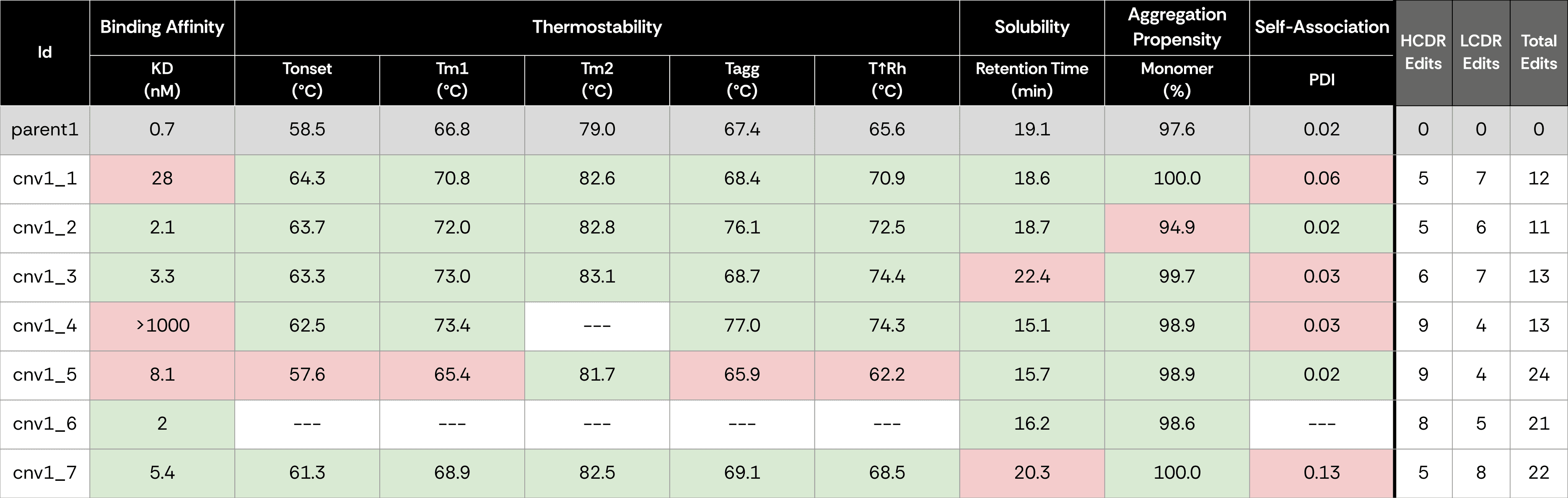
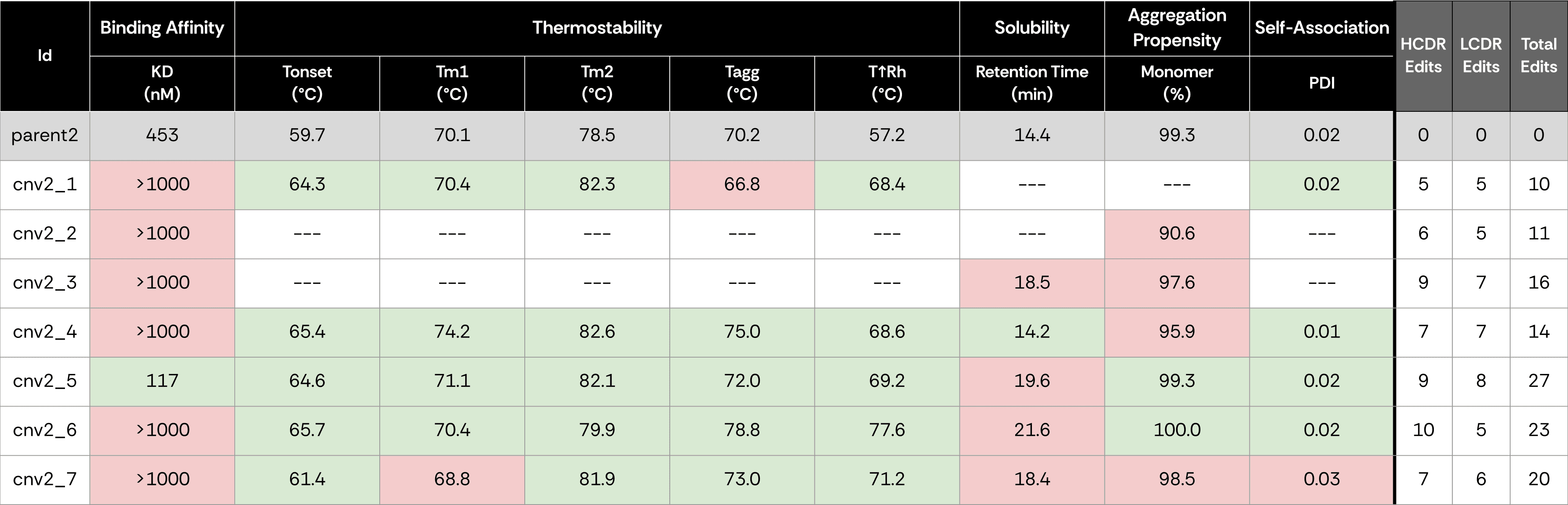
We chose two published anti–SARS-CoV-2 IgGs, one exhibiting high binding affinity and the other medium affinity, as parental antibody sequences, to undergo IP expansion.
We applied ConvergeAB™ to computationally generate seven novel antibody variants (Cnv1–Cnv7) for each parent, exhibiting substantial sequence divergence from the parental clone.
All antibodies, including the two parental sequences, were expressed in IgG format, purified and characterized side-by-side across five key assays: Binding affinity was measured by Surface Plasmon Resonance (SPR), Thermostability was measured by Nano Differential Scanning Fluorimetry (nanoDSF), Solubility was measured by Hydrophobic Interaction Chromatography (HIC), Aggregation propensity was measured by analytical Size-Exclusion Chromatography (aSEC), and Self-association was measured by Dynamic Light Scattering (DLS).
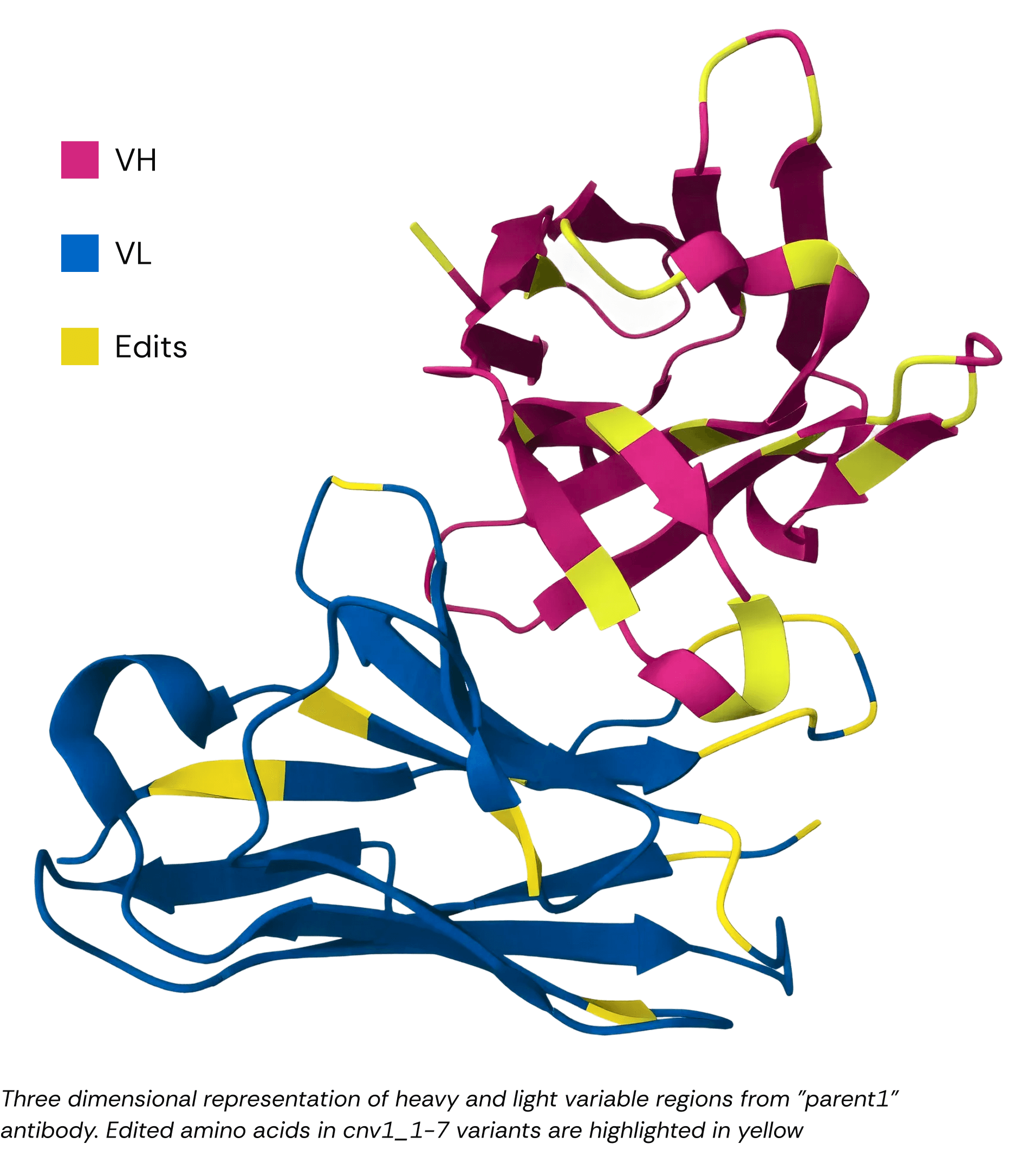
Results
For the high affinity binder (parent1), six of the seven generated variants (86%) demonstrated specific binding to the SARS-CoV-2 antigen. Two antibodies had affinities comparable to the original antibody (<3nM), and three more had single digit nanomolar affinities. Most antibodies displayed equal or improved developability traits, validating ConvergeAB™’s capacity to simultaneously optimize multiple dimensions of antibody quality. For the medium affinity binder (parent2), one of the seven variants (15%) showed specific binding to the antigen with 4-fold better binding affininty, and had overall favorable developability traits.
Scientific Insight: From Reactive to Generative
ConvergeAB™’s generative design engine explored mutational landscapes beyond human intuition. This approach produces sequence diversity that maintains core biophysical properties while opening new IP frontiers.
“Generative AI allows us to look at antibody design not as an optimization exercise, but as the exploration of entirely new molecular possibilities.”
—
Dov Gertz, CEO & Co-Founder at Converge Bio
This capability signifies a shift from reactive antibody improvement to proactive biologic creation, where every design cycle strengthens both the scientific and business potential of a molecule portfolio.
Economic and Operational Impact
1. Portfolio-Level IP Expansion
By taking an existing antibody, ConvergeAB™ is able to generate sequences that preserve or improve their target binding affinity, despite making significant changes in the CDR sequences (11-22 amino acids), all while preserving or improving on the technical attributes of the antibody (thermostability, solubility, monomer content, etc.). With that, ConvergeAB™ delivers substantially divergent antibody variants that are:
Patentable as new molecular entities
More defensible from an IP standpoint
Ready for further engineering or optimization
For pharma and biotech teams, this means broader, stronger pipeline optionality without proportional increases in experimental cost.
2. Accelerated R&D Timelines
By enabling in silico prediction and filtering before lab validation, ConvergeAB™ can potentially reduce experimental iterations significantly, cutting both time-to-validation and associated costs.
3. Improved Manufacturability and Risk Reduction
Variants exhibiting improved stability and solubility reduce manufacturing risk and batch failure rates, leading to more predictable production yields and lower COGS. These improvements translate to material reductions in CMC risk, especially in scaling campaigns.
4. Platform Generalizability & Scalability
This study was performed without target-specific model tuning, demonstrating that ConvergeAB™ can:
Be applied immediately to new targets
Accelerate antibody programs with limited initial data
Support both early discovery and later-stage optimization
This maximizes ROI for partners using Converge Bio’s platform across multiple therapeutic areas.
Broader Significance: The Future of Biologic Innovation
The SARS-CoV-2 study is a demonstration of how Converge Bio’s AI-driven frameworks enable organizations to achieve both scientific excellence and operational efficiency.
ConvergeAB™ proves that artificial intelligence can do more than assist R&D - it can reshape how intellectual property, cost efficiency, and discovery velocity intersect.
Conclusion
ConvergeAB™’s SARS‑CoV‑2 study demonstrates how generative AI can unlock new therapeutic candidates, reduce discovery cost, and de-risk development. For pharma and biotech companies, this represents a new class of capability: scalable, design-first innovation that strengthens both scientific and business outcomes.
Click here to learn more about ConvergeAB™.
Click here to book a meeting with the Converge team.


