
Iddo Weiner
|
Feb 16, 2026
Background: Internal benchmarking initiative.
Goal: Assess the biophysical properties and developability of ConvergeAB™ generated antibodies.
Approach: Generative multiparameter optimization to known antibodies involving substantial sequence editing compared against FDA-approved clinical standards.
Outcome: Generated antibodies demonstrated biophysical profiles indistinguishable from blockbuster drugs (e.g., Keytruda, Humira) in the first iteration, significantly streamlining the lead optimization process.
---
The Context & Challenge
In antibody discovery, identifying a molecule that binds to a target is a critical first step, but transforming that molecule into a viable drug presents a significant downstream challenge. Developability - ensuring a molecule is stable, soluble, and manufacturable - is often a laborious and time-consuming bottleneck in drug development.
Traditionally, antibodies discovered through screening or early-stage design may suffer from poor biophysical traits, requiring extensive cycles of "lead optimization" to fix issues like aggregation or low thermostability. The challenge we addressed was fundamental: Can a generative AI platform produce antibodies that are "developability-ready" from the get-go, making the early discovery phase far more efficient?
The Solution: Benchmarking Against the Best
To test this, we designed an internal benchmark comparing ConvergeAB™ candidates directly against the industry's "gold standard": FDA-approved clinical antibodies.
The Cohort: We generated 64 IgG antibodies, based on a number of known antibodies against various targets using ConvergeAB™. Significant changes were made to these antibodies in order to improve their biophysical profiles. Importantly, the platform generated these sequences without relying on the specific clinical controls used in the comparison.
The Controls: We compared these against 12 blockbuster clinical antibodies, including Adalimumab (Humira), Pembrolizumab (Keytruda), Cetuximab, and Atezolizumab.
The Metrics: All molecules were tested side-by-side for Thermostability (Tm) via nanoDSF (an indicator of greater structural stability and developability) and Solubility/Hydrophobicity via HIC (Hydrophobic Interaction Chromatography).
Results
ConvergeAB™ generated antibodies that are biophysically equivalent to clinical standards in the first iteration.
The analysis reveals a similar distribution between the two groups. The majority of the AI-generated antibodies (44/64, 69%) fall within the distribution of the clinical controls (10/12, 83%), specifically clustering in areas associated with favorable traits and high developability.
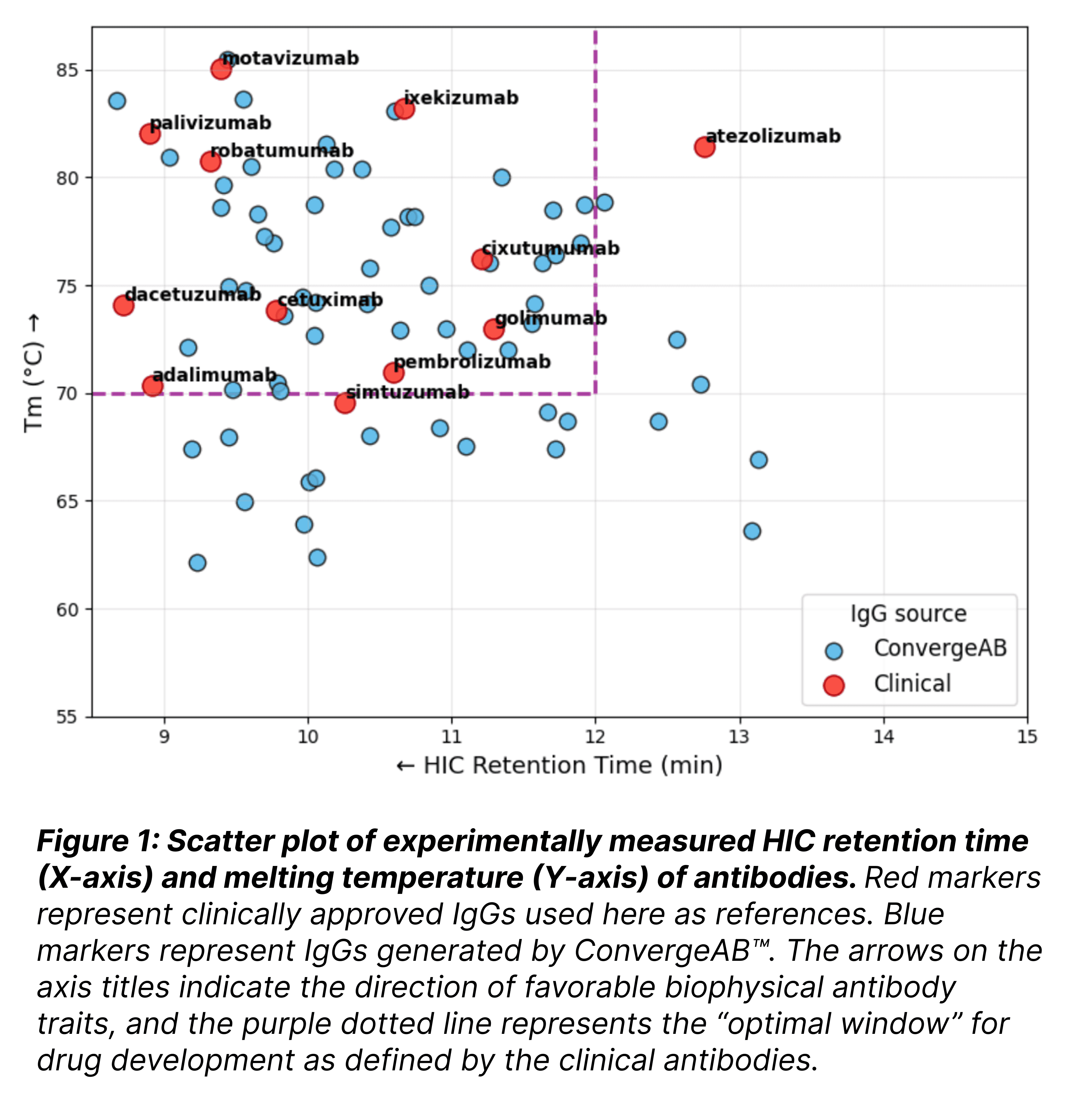
Key observations include:
Clinical Equivalence: The AI-generated candidates fall within the same "optimal window" (top-left quadrant) as major drugs like Palivizumab and Atezolizumab, exhibiting high melting temperatures (>70°C) and favorable solubility profiles.
The hit-rate of the clinical antibodies within this optimal window was 10 out of 12 antibodies (83%), compared to ConvergeAB's™ 44 out of 64 (69%).
Zero-Shot Success: Without ever "seeing" these specific clinical benchmarks during training, the platform successfully generated scaffolds that adhere to the strict biophysical rules required for a therapeutic drug.
Scientific Insight
These results prove that ConvergeAB™ does not just optimize for binding; it implicitly learns the structural rules of protein stability. By generating molecules that effectively "pre-solve" developability challenges, the platform ensures that candidates are not just active, but physically robust enough to endure the manufacturing process.
Conclusion: De-risking Development from Day One
This study confirms that ConvergeAB™ generates clinic-ready molecules immediately, offering a massive advantage in the drug development lifecycle.
For biopharma partners, this translates into significant downstream value:
More Efficient Lead Optimization: By starting with antibodies that already match the developability profile of drugs like Keytruda, R&D teams can focus their optimization efforts on other critical properties—such as potency, selectivity, and pharmacokinetics—rather than spending months fixing fundamental stability issues.
De-risking CMC: Process development teams inherit "easier" antibodies with high stability and solubility, significantly reducing the risk of batch failures or formulation challenges later in the pipeline.
Accelerated Discovery: By solving for manufacturability at the moment of generation, we bridge the gap between early discovery and clinical reality, allowing programs to move faster with higher confidence.
Click here to learn more about ConvergeAB™.
Click here to contact the Converge team.


